Parent company Rhegen Biotech's lyophilized mRNA vaccine for tuberculosis (RH119) has received CDE approval for clinical trial application.
#Rhegen Biotech's Lyophilized Tuberculosis mRNA Vaccine Clinical Trial Application Approved by CDE#
Recently, the clinical trial application (IND) for the lyophilized tuberculosis mRNA vaccine independently developed by Shenzhen Rhegen Biotech Co., Ltd. has received implied permission from the Center for Drug Evaluation (CDE) of the National Medical Products Administration of China. This vaccine is the world's first lyophilized tuberculosis mRNA vaccine with independent intellectual property rights.
The vaccine, designated RH119, is a lyophilized injectable preparation targeting tuberculosis caused by Mycobacterium tuberculosis. It is classified as a 1.1 class new drug (vaccine for diseases without effective prevention methods). The vaccine aims to prevent healthy populations from being infected with Mycobacterium tuberculosis, prevent the onset of tuberculosis in latent infections and close contacts, and improve treatment outcomes.
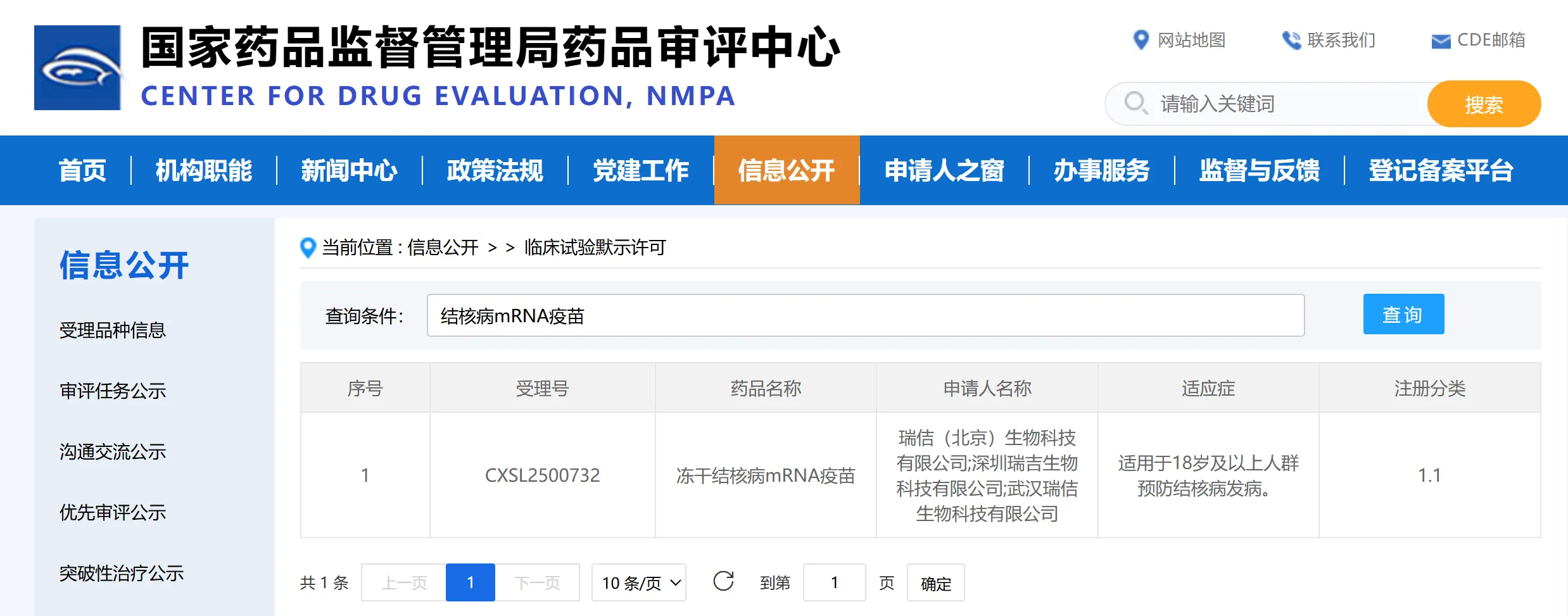 Prior to this IND approval, Rhegen Biotech had already conducted two key investigator-initiated trials (IIT). In January 2025, the company collaborated with Wuhan Jinyintan Hospital to conduct an IIT clinical study (ChiCTR2400094049) to evaluate the safety and immunogenicity of RH119 in healthy adult subjects aged 18-59. In March 2025, Rhegen Biotech again collaborated with Beijing Chest Hospital to conduct a similar IIT study targeting adult latent tuberculosis infections. Preliminary data from both trials showed that RH119 exhibited extremely high safety and strong immunogenicity, inducing high levels of antigen-specific cellular immune responses, which are key to clearing Mycobacterium tuberculosis and blocking infection progression.
Notably, RH119 adopts a lyophilized formulation, which significantly facilitates vaccine storage, transportation, and grassroots application. Compared to the strict cold chain requirements of traditional liquid vaccines, lyophilized vaccines can better adapt to medical infrastructure in different regions worldwide, especially in tuberculosis high-burden developing countries, greatly reducing the threshold for vaccine popularization and laying the foundation for subsequent global promotion.
Tuberculosis remains a major global public health problem. According to WHO data, approximately one-quarter of the global population is infected with Mycobacterium tuberculosis, with about 2-3 people dying from tuberculosis every minute. Since 1921, BCG vaccine has been widely used for tuberculosis prevention, but it has significant limitations: its protective effect lasts only about 15 years, gradually decreasing with time, and its protective effect on adolescents and adults is poor. Therefore, developing more effective tuberculosis vaccines is an urgent global health need.
Rhegen Biotech is a biotechnology company with globally leading mRNA technology. Since its establishment in 2019, the company has built a technology platform covering the entire mRNA field process, mastered unique underlying original technologies, and accumulated multiple core patents. Through continuous product development and technological innovation, it has formed a technology platform and capabilities covering the entire chain with independent intellectual property rights and druggability characteristics, applied to fields with huge market prospects and clinical value such as tumors, autoimmune diseases, metabolism, and major infectious diseases.
Prior to this IND approval, Rhegen Biotech had already conducted two key investigator-initiated trials (IIT). In January 2025, the company collaborated with Wuhan Jinyintan Hospital to conduct an IIT clinical study (ChiCTR2400094049) to evaluate the safety and immunogenicity of RH119 in healthy adult subjects aged 18-59. In March 2025, Rhegen Biotech again collaborated with Beijing Chest Hospital to conduct a similar IIT study targeting adult latent tuberculosis infections. Preliminary data from both trials showed that RH119 exhibited extremely high safety and strong immunogenicity, inducing high levels of antigen-specific cellular immune responses, which are key to clearing Mycobacterium tuberculosis and blocking infection progression.
Notably, RH119 adopts a lyophilized formulation, which significantly facilitates vaccine storage, transportation, and grassroots application. Compared to the strict cold chain requirements of traditional liquid vaccines, lyophilized vaccines can better adapt to medical infrastructure in different regions worldwide, especially in tuberculosis high-burden developing countries, greatly reducing the threshold for vaccine popularization and laying the foundation for subsequent global promotion.
Tuberculosis remains a major global public health problem. According to WHO data, approximately one-quarter of the global population is infected with Mycobacterium tuberculosis, with about 2-3 people dying from tuberculosis every minute. Since 1921, BCG vaccine has been widely used for tuberculosis prevention, but it has significant limitations: its protective effect lasts only about 15 years, gradually decreasing with time, and its protective effect on adolescents and adults is poor. Therefore, developing more effective tuberculosis vaccines is an urgent global health need.
Rhegen Biotech is a biotechnology company with globally leading mRNA technology. Since its establishment in 2019, the company has built a technology platform covering the entire mRNA field process, mastered unique underlying original technologies, and accumulated multiple core patents. Through continuous product development and technological innovation, it has formed a technology platform and capabilities covering the entire chain with independent intellectual property rights and druggability characteristics, applied to fields with huge market prospects and clinical value such as tumors, autoimmune diseases, metabolism, and major infectious diseases.
News you may be interested in:


