In the development of mRNA-based drugs and vaccines, modified nucleosides play a critical role. By replacing natural nucleosides (such as uridine) with specific chemically modified analogs (e.g., pseudouridine, N1-methylpseudouridine, 5-methoxyuridine, etc.), the druggability of mRNA molecules can be significantly enhanced. Key improvements include effectively reducing immunogenicity to prevent excessive inflammatory responses, increasing translation efficiency to yield higher levels of the target protein, and improving molecular stability to extend its in vivo half-life. Research and application of modified nucleosides represent one of the key breakthroughs that enabled the transition of mRNA technology from concept to clinical reality.

ENO Bio has established foundational capabilities in the design and synthesis of modified nucleosides. This enables the provision of an extensive library of modified nucleosides for partners, encompassing a wide range of commonly used and novel types (e.g., pseudouridine, N1-methylpseudouridine, 5-methoxyuridine, thio-modified nucleosides, etc.). Leveraging this platform, ENO Bio collaborates with partners to explore novel modification strategies (such as double modifications and labeled modifications), aiming to achieve finer control over mRNA immunostimulatory properties, translation kinetics, and targeted delivery efficiency. Furthermore, ENO Bio supports partners in developing manufacturing processes and conducting regulatory filings for Active Pharmaceutical Ingredients (APIs) of custom-modified nucleosides, addressing the evolving demands for enhanced druggability in broader clinical applications.
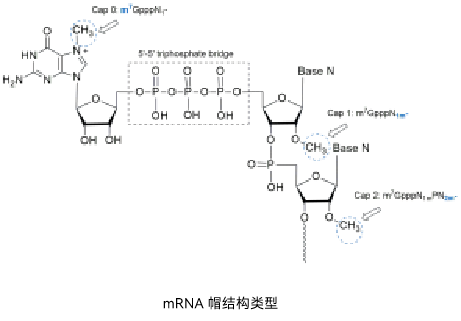
Recognizing the critical importance of the 5' cap, ENO Bio has also built core capabilities in the synthesis and evaluation of cap analogs. The platform reliably produces high-quality standard cap analogs, such as Cap1 analogs, meeting essential needs for mRNA product development. This foundation has been expanded to include custom synthesis and functional analysis of diverse cap structures, assisting clients in exploring both typical and atypical novel cap architectures for mRNA pharmaceuticals. Significantly, ENO Bio has constructed a proprietary library of tissue-specific Cap2 analogs, termed RHCaps (covered by PCT patents including PCT/CN2020/124720, PCT/CN2024/073220, PCT/CN2024/085861). mRNA synthesized using RHCaps demonstrates significantly improved druggability. Depending on the specific RHCap structure used, the mRNA exhibits distinct tissue specificity, potentially leading to enhanced cellular immune responses, higher target tissue protein expression levels, and reduced off-target delivery effects. This technology provides clients with a novel option to address druggability challenges in a wider range of therapeutic areas while achieving intellectual property breakthroughs.