
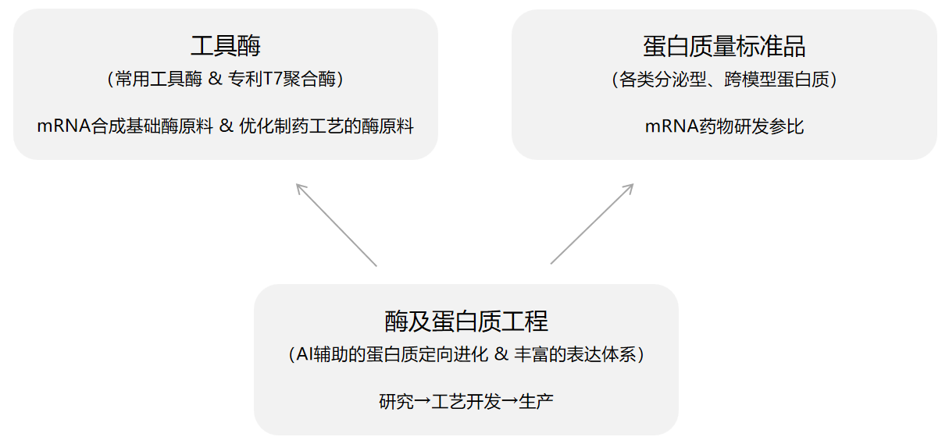
Enzymes and Protein Engineering
ENO Bio has established foundational capabilities in the design and synthesis of modified nucleosides. This platform enables the provision of an extensive library of modified nucleosides for partners, encompassing a wide range of commonly used and novel types (e.g., pseudouridine, N1-methylpseudouridine, 5-methoxyuridine, thio-modified nucleosides). Leveraging this capability, ENO Bio collaborates with partners to explore novel modification strategies (such as double modifications and labeled modifications), facilitating precise modulation of mRNA immunostimulatory properties, translation kinetics, and targeted delivery efficiency. Furthermore, ENO Bio supports partners in developing manufacturing processes and conducting regulatory filings for Active Pharmaceutical Ingredients (APIs) of custom-modified nucleosides, addressing the evolving demands for enhanced druggability in broader clinical applications.
Recognizing the critical importance of the 5' cap, ENO Bio has built core capabilities in the synthesis and evaluation of cap analogs. The platform reliably produces high-quality standard cap analogs, including Cap1 analogs, meeting essential requirements for mRNA product development. This foundation has been expanded to include custom synthesis and functional analysis of diverse cap structures, assisting clients in exploring both typical and atypical novel cap architectures for mRNA pharmaceuticals. Significantly, ENO Bio has constructed a proprietary library of tissue-specific Cap2 analogs, termed RHCaps (covered by PCT patents including PCT/CN2020/124720, PCT/CN2024/073220, PCT/CN2024/085861). mRNA synthesized using RHCaps demonstrates significantly improved druggability. Depending on the specific RHCap structure, the resulting mRNA exhibits distinct tissue specificity, potentially leading to enhanced cellular immune responses, higher target tissue protein expression, and reduced off-target delivery effects. This technology provides clients with a novel strategic option to address complex druggability challenges across a wider range of therapeutic areas while achieving meaningful intellectual property breakthroughs.
ENO Bio has developed an engineered T7 RNA polymerase mutant through a combination of machine learning and directed mutagenesis. Using various cap analogs for screening, the enzyme was specifically evolved for high capping efficiency, reduced dsRNA production, increased yield, and enhanced thermostability. The resulting mutant demonstrates broad compatibility with both standard Cap1 analogs and ENO Bio's proprietary Cap2 analogs (RHCaps).
This novel T7 polymerase mutant exhibits a capping rate exceeding 95% for both commercially available Cap1 analogs and ENO Bio's proprietary RHCaps. It shows significantly improved thermal stability, with peak activity at 50°C reaching up to 2.5 times that of the wild-type (WT) enzyme at 37°C. The mutant also achieves substantially higher IVT yields compared to commercially available thermostable T7 polymerases, while generating only 25% of the dsRNA produced by the WT enzyme.